Pyrimidine Metabolism
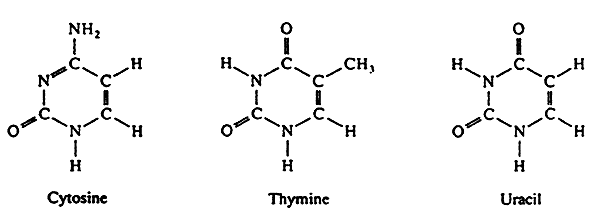
The Bases
Pyrimidines:

Pyrimidine Biosynthesis
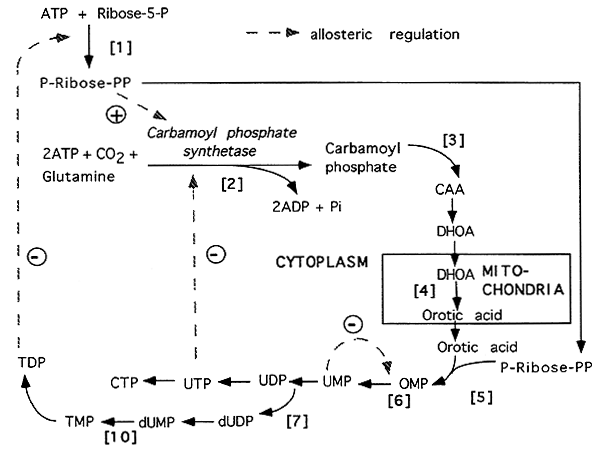
Note: CAA = Carbamoyl Aspartate; DHOA = Dihydroorotate; OMP = Orotidine Monophosphate
[1] PRPP Synthetase (see "Purine Lecture")
[2] Carbamoyl Phosphate Synthetase II (glutamine):
Glutamine +
CO2 + 2 ATP ----> Carbamoyl phosphate + Glutamate + 2 ADP + Pi
Carbamoyl Phosphate Synthetase II (CPS-II) differs in several ways from its isoform (CPS-I), the enzyme which provides carbamoyl phosphate for the Urea cycle (see "Protein Turnover / Ammonia Metabolism", CHE 452).
1. CPS-II is located in the cytoplasm, CPS-I mitochondria
2. Glutamine not Ammonia is the Nitrogen source
3. CPS-II does not require N-acetylglutamate as an allosteric activator
4. CPS-I is found only in Liver and Kidney, CPS-II in most tissues
[3] Aspartate Carbamoyl Transferase: the committed
step
Carbamoyl phosphate + Aspartate ----> Carbamoyl aspartate + Pi
[4] Dihdroorotate Dehydrogenase: the only Mitochondrial
enzyme in the pathway
Dihydroorotate + NAD+ ----> Orotate + NADH + H+
[5] Orotate Phosphoribosyltransferase: Orotate + PRPP ----> OMP + PPi
[6] OMP Decarboxylase: OMP ----> UMP + CO2
OMP is the first pyrimidine formed and is immediately decarboxylated to produce
UMP. Nucleotides are then formed subsequently from UTP via CTP Synthetase.
[7] Synthesis of Thymidine nucleotides first requires deoxyribonucleotide synthesis. The enzyme responsible for this step is Ribonucleotide Reductase. This enzyme acts on oxynucleotides in their diphosphate form. Thioredoxin, a small protein, is oxidized as the 2' hydroxyl group on the ribose ring is reduced. Oxidized Thioredoxin (S-S) is then reduced by FADH2 and NADPH. The products are the respective deoxynucleotide diphosphates which are further phosphorylated and then used for DNA synthesis.
The exception dUDP is first converted to thymidine nucleotide via dUMP (see [10] below).
[8] A deficiency of Adenosine Deaminase (ADA) decreases metabolism of deoxyadenosine causing dATP to accumulate.
[9] Similarly a deficiency of Purine Nucleoside Phosphorylase causes accumulation of dGTP. In both cases Ribonucleotide Reductase is inhibited by accumulation of these nucleotides.
[10] Synthesis of thymidylate requires uridine nucleotide
to be converted to the deoxy form. The substrate for Thymidylate Synthetase
is dUMP, the N5,N10-methylene-tetrahydrofolate (CH3-THF)
is the "methyl" donor.
This form of folate derives its carbon from serine: Serine + THF ----> Glycine
+ CH3-THF
Regulation of Pyrimidine Synthesis
The primary site of regulation is Carbamoyl Phosphate Synthetase II which is allosterically inhibited by UTP. Elevated PRPP increases the CPS-II activity to help control PRPP levels. Feedback inhibition (control) is provided by TDP inhibition of PRPP synthesis and UMP inhibition of OMP Decarboxylase.
Clinical Correlate: Chemotherapy
5-Fluorouracil: is a pyrimidine analog which is converted metabolically to its toxic form, fluorodeoxyuridylate (F-dUMP). As cells metabolically activate the drug it acts as a "suicide" inhibitor of Thymidylate Synthetase.
Methotrexate (MTX, amethopterin): is a folic acid analog used to inhibit Dihydrofolate Reductase. Since the thymidine nucleotide requirements of rapidly proliferating cells are much greater than for quiescent cells folinic acid cannot meet the demands of the cancer cells.
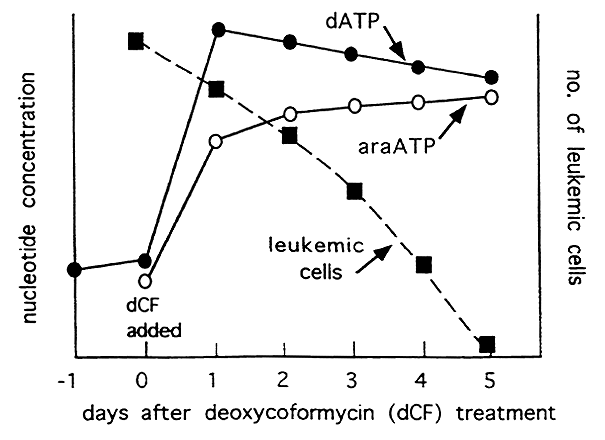
Deoxycoformycin inhibits Adenosine Deaminase to increase the levels of dATP which inhibits Ribonucleotide Reductase. Infusing Adenosine Arabinoside together with Deoxycoformycin increases inhibition of the Reductase further via elevated levels of both dATP and arabinoside-ATP. Since deoxynucleotides are essential for DNA synthesis this strategy is effective in treating certain types of leukemia.
© Dr. Noel Sturm 2014