BREAKTHROUGH OF THE YEAR:
Small RNAs Make Big Splash
Jennifer Couzin
#1 The Winner
Just when scientists thought they
had deciphered the roles played by the cell's leading actors, a familiar
performer has turned up in a stunning variety of guises. RNA, long upstaged by
its more glamorous sibling, DNA, is turning out to have star qualities of its
own.
|
See |
For decades, RNA molecules were dismissed as little more than
drones, taking orders from DNA and converting genetic information into proteins.
But a string of recent discoveries indicates that a class of RNA molecules
called small RNAs operate many of the cell's controls. They can turn the tables
on DNA, shutting down genes or altering their levels of expression. Remarkably,
in some species, truncated RNA molecules literally shape genomes, carving
out chunks to keep and discarding others. There are even hints that certain
small RNAs might help chart a cell's destiny by directing genes to turn on
or off during development, which could have profound implications for coaxing
cells to form one type of tissue or another. Science hails these
electrifying discoveries, which are prompting biologists to overhaul their
vision of the cell and its evolution, as 2002's Breakthrough of the Year.
Life cycle. With a helping hand from proteins RISC and Dicer,
small RNAs are born. We now know that these molecules keep DNA in line and
ensure a cell's good health.
ILLUSTRATION: C. SLAYDEN/G. RIDDIHOUGH
These astonishing feats are performed by short stretches of RNA ranging in
length from 21 to 28 nucleotides. Their role had gone unnoticed until recently,
in part because researchers, focused on the familiar larger RNA molecules,
tossed out the crucial small ones during experiments. As a result, RNA has long
been viewed primarily as an essential but rather dull molecule that ferries the
genetic code from the nucleus to the ribosomes, the cell's protein factories,
and helps assemble amino acids in the correct order during protein synthesis.
Signs that RNA might be more versatile came in the early 1990s, when
biologists determined that some small RNAs could quash the expression of
various genes in plant and, later, animal cells. But they didn't appreciate the
molecules' true powers until 1998. That's when Andrew Fire of the Carnegie
Institution of Washington in Baltimore, Maryland, Craig Mello of the University
of Massachusetts Medical School in Worcester, and their colleagues injected
stretches of double-stranded RNA into worms. Double-stranded RNA forms when a
familiar single strand kinks back in a hairpin bend, putting two complementary
sequences alongside each other. To the researchers' surprise, double-stranded
RNA dramatically inhibited genes that had helped generate the RNA in the first
place. This inhibition, which was later seen in flies and other organisms, came
to be known as RNA interference (RNAi). It helped prove that RNA molecules were
behind some gene silencing.
Another crucial step came last year, when Gregory Hannon of Cold Spring
Harbor Laboratory in New York and his colleagues identified an enzyme,
appropriately dubbed Dicer, that generates the small RNA molecules by chopping
double-stranded RNA into little pieces. These bits belong to one of two small
RNA classes produced by different types of genes: microRNAs (miRNAs) and small
interfering RNAs (siRNAs). SiRNAs are considered to be the main players in
RNAi, although miRNAs, which inhibit translation of RNA into protein, were
recently implicated in this machinery as well.
To bring about RNAi, small RNAs degrade the messenger RNA that transports a
DNA sequence to the ribosome. Exactly how this degradation occurs isn't known,
but scientists believe that Dicer delivers small RNAs to an enzyme complex
called RISC, which uses the sequence in the small RNAs to identify and degrade
messenger RNAs with a complementary sequence.
Such degradation ratchets down the expression of the gene into a protein.
Although quashing expression might not sound particularly useful, biologists
now believe that in plants, RNAi acts like a genome "immune system,"
protecting against harmful DNA or viruses that could disrupt the genome.
Similar hints were unearthed in animals this year. In labs studying gene
function, RNAi is now commonly used in place of gene "knockouts":
Rather than delete a gene, a laborious process, double-stranded RNA is applied
to ramp down its expression.
The year's most stunning revelations emerged in the fall, in four papers
examining how RNA interference helps pilot a peculiar--and pervasive--genetic
phenomenon known as epigenetics. Epigenetics refers to changes in gene
expression that persist across at least one generation but are not caused by
changes in the DNA code.
In recent years, researchers have found that one type of epigenetic regulation
is caused by adjustments in the shape of complexes known as chromatin, the
bundles of DNA and certain fundamental proteins that make up the chromosomes.
By changing shape--becoming either more or less compact--chromatin can alter
which genes are expressed. But what prompts this shape-shifting remained
mysterious.
This year, scientists peering closely at RNAi in two different organisms
were startled to find that small RNAs responsible for RNAi wield tremendous
control over chromatin's form. In so doing, they can permanently shut down or
delete sections of DNA by mechanisms not well understood, rather than just
silencing them temporarily.
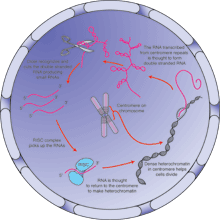
That news came from several independent groups. In one case, Shiv Grewal,
Robert Martienssen, and their colleagues at Cold Spring Harbor Laboratory
compared fission yeast cells lacking RNAi machinery with normal cells. When
yeast cells divide, their chromosomes untangle and migrate to opposite sides of
the cell. The researchers already knew, broadly, that this chapter of cell
division is governed by a tightly wrapped bundle of chromatin, called
heterochromatin, around the centromere--the DNA region at the chromosome's
"waist." The biologists found that their mutant cells, which were
missing the usual small RNAs, couldn't properly form heterochromatin at their
centromeres and at another DNA region in yeast that controls mating. This
suggests that without small RNAs, cell division goes awry. The scientists
theorized that in healthy yeast cells, small RNAs elbow their way into cell
division, somehow nudging heterochromatin into position to do the job. That
exposes DNA to different proteins and dampens gene expression.
Meanwhile, David Allis and his colleagues at the University of Virginia
Health System in Charlottesville, along with Martin Gorovsky of the University
of Rochester in New York and others, were focusing on a different organism, a
single-celled ciliate called Tetrahymena. Biologists treasure Tetrahymena
because it stores the DNA passed to offspring in a different nucleus from the
one containing DNA expressed during its lifetime, making it easy to distinguish
one gene set from the other. The researchers found that in Tetrahymena,
small RNAs trigger deletion or reshuffling of some DNA sequences as a cell
divides. RNAi appeared to be targeting structures analogous to heterochromatin,
only this time strips of DNA were discarded or moved elsewhere. The mechanism
remains unclear, however.
The two sets of experiments might help explain why small RNAs exist in the
first place. In both the yeast and Tetrahymena, small RNAs' frenetic
activity is focused on genome regions, such as centromeres, that contain
repetitive DNA resulting from transposons. Transposons are bits of DNA that can
jump around the genome and insert themselves at different locales; at times,
they jam transcription machinery and cause disease. It appears
possible--although still largely hypothetical--that small RNAs evolved very
early in life's history to help protect the genome against instability.
This is just one of many areas that remain to be explored. Researchers are
still trying to sort out how the well over 100 different miRNAs function and
which species contain which ones. There are hints that they behave differently
in plants and animals. And some recent work suggests that miRNAs exert more
control over gene expression than previously believed. Also a focus of research
are the proteins, such as Dicer, that are critical cogs in the RNAi machinery.
Researchers are also probing RNAi's possible role in development and
disease. RNAi has been implicated in guiding meristems, the plant version of
stem cells, so some biologists believe that it might help establish the path
taken by human and other mammalian stem cells as they differentiate into certain
tissues. If so, RNAi could prove an essential tool in manipulating stem cells.
And if small RNAs influence cell division in humans as they do in yeast and Tetrahymena,
minor disruptions in the machinery could lead to cancer.
The extraordinary, although still unfulfilled, promise of small RNAs and
RNAi has split the field wide open and put RNA at center stage. Having exposed
RNAs' hidden talents, scientists now hope to put them to work.