Glycogen Metabolism
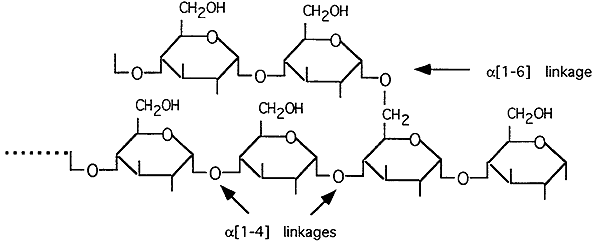
Glycogen

Alpha 1,4-glycosidic linkages predominate, 1,6-glycosidic linkages form the branches.
Glycogenolysis:
Glycogen Phosphorylase:
-Catalyzes the the removal of glucose units from the ends of branches.
-Contains Binding Sites For:
substrates (glycogen, Pi)
co-factor (pyridoxal phosphate)
allosteric activator (AMP)
allosteric inhibitors (ATP, glucose, glucose-6-phosphate)
-Active Form, "a", phosphorylated
-Inactive Form, "b", dephosphorylated
Regulation: complex, to ensure glucose remains stored as glycogen until absolutely required to maintain blood glucose homeostasis or to supply energy to the cell
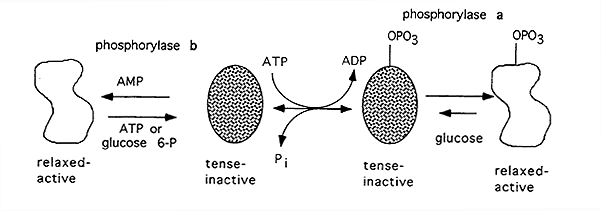
-Whether or not it is phosphorylated it can exist in a "tense"(inactive) form or a "relaxed" (active) form (only high glucose can force the existence of the phosphorylated tense form).
-The enzyme can be rapidly activated without undergoing phosphorylation in response to a hormonal signal
Activator: AMP
Inhibitor: ATP, glucose, glucose-6-phosphate
Glycogenesis:
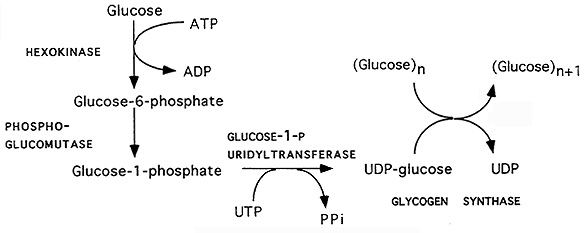
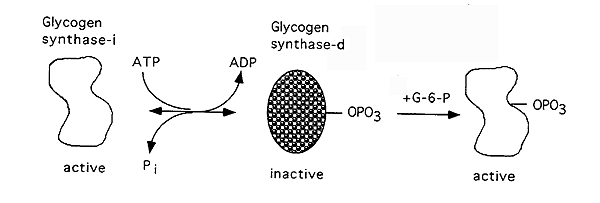
Glycogen Synthase Regulation: opposes that of glycogen phosphorylase
Active, dephosphorylated
Inactive, phosphorylated
Glycogen Synthase-i: independent (i) of glucose-6-phosphate for its activity.
Glycogen Synthase-d: dependence (d) on glucose-6-phosphate, mechanism for storing glucose when overabundance is signalled by a build-up of glucose-6-phosphate.
© Dr. Noel Sturm 2014