Gas Transport
Hemoglobin Structure
40 Structure of Hb:
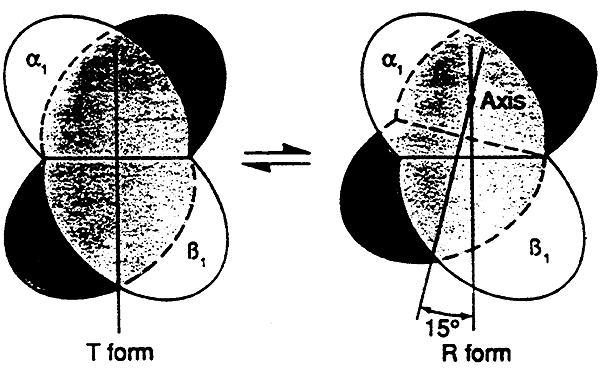
Oxy-Hb is referred to as being in the relaxed(R) state
Deoxy-Hb is referred to as being in the tense(T) state

These designations, R and T, were introduced by Jacques Monod, and refer to relaxed and tense forms of all allosteric proteins.
"The R form has a higher affinity for oxygen than the T form, whether or not oxygen is present."
Orientation of Hemoglobin Subunits:
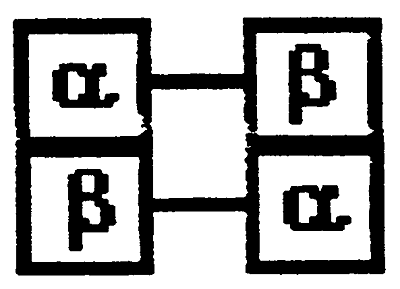
Hemoglobin is actually a dimer of dipeptides.
When oxygen binds we have Oxyhemoglobin (R)- one pair of the dipeptides rotates, opening a 150 cleft
Deoxyhemoglobin (T)- two pairs sit in close contact (as in the pisture above)
The story of the Salt Bridges:
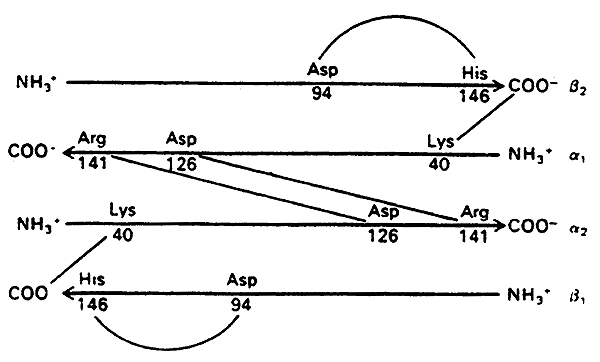
DeoxyHb (T state)- stabilized by salt bridges between the chains
When oxygen binds OxyHb (R state)- the salt bridges are disrupted
Hence Oxygen binding induces a conversion from the T to the R state, increasing affinity for itself (cooperativity).
Cooperativity:
Binding one molecule of O2 enhances binding the subsequent O2 molecules.
When one O2 binds it provokes a T to R conversion on the other subunits.
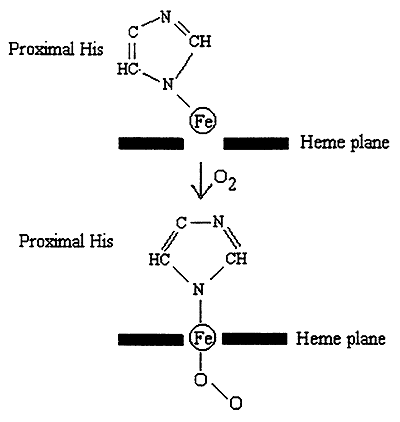
1. Bound O2 "pulls" the Iron atom into the plane of the heme.
2. Movement of the Iron pulls the proximal His.
3. Movement of His affects the structure ---> disrupts salt bridges.
4. This "frees up" another subunit, which now exists in an R-like state with increased affinity for O2.
5. Binding of O2 to the second subunit triggers the same sequence, enhancing binding to the third subunit etc.....
Protons (H+), CO2, 2,3-bisphosphoglycerate (BPG) (in tissues): induce a conversion from the R to the T state, decreasing affinity for oxygen and causing it to be released to the tissues.
Bohr (Proton) Effect:
O2 binding to Hb is sensitive to physiological pH changes.
i.e. Contracting Muscle- lactic acid is produced, hence the H+ concentration increases, pH decreases -------->promotes O2 dissociation (fulfills greater requirement for O2).
H+ antagonizes the binding of O2 to Hb.
In lungs, higher pH increases affinity of Hb for O2 (reverse of what is happening in muscle).
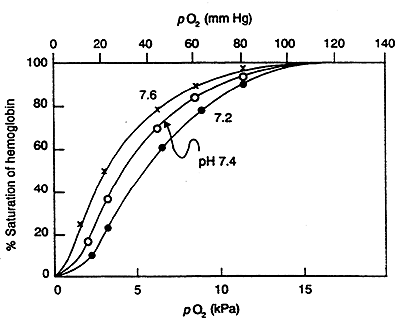
In terms of R and T:
H+ induces an R to T conversion, His becomes protonated giving it a (+) charge, induces salt bridge formation with Asp a characteristic of the T state.
Carbon Dioxide:
CO2 is a byproduct of metabolism.
Accumulation of CO2 is a characteristic of active metabolism and increased O2 requirements by the tissue.
CO2 lowers the affinity of Hb for O2 so that in the tissues O2 is released.
In lungs CO2 is kept lower by expiration increasing the affinity of Hb for O2 (reverse of what is happening in tissues).
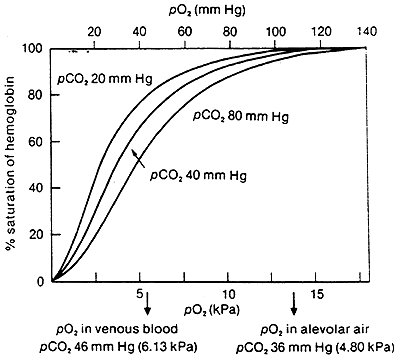
In terms of R and T:
CO2 binds to the N-terminus of Hb, HbNH-COO-, this forms a salt bridge stabilizing the T state.
2,3-Bisphosphoglycerate:
BPG is produced in the RBC from the metabolism of glucose
BPG lowers the affinity of Hb for O2
Accumulation of BPG is a characteristic of active metabolism and increased O2 requirements by the tissue.
In lungs, when O2 is bound, the cavity where BPG binds becomes to small, thus the increased affinity for O2 in the lungs.
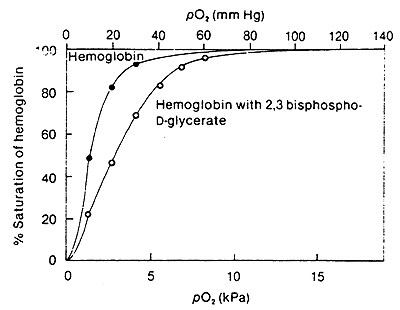
In terms of R and T:
The (-) BPG interacts with (+) charged residues (Lys, His etc.) cross-linking the b- subunits.
Again these interactions stabilize the T state.
Clinical Correlate:
Fetal Hemoglobin
a2g2 tetramer instead of a2b2
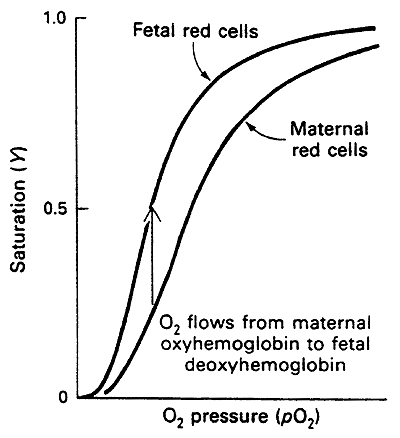
In the gamma (g) subunit much of the His becomes Ser
The lack of His disrupts salt bridge formation
Hence HbF is more relaxed and has a higher affinity for O2 than adult Hb
This higher affinity optimizes the transfer of O2 from maternal to fetal circulation thus oxygenating the fetus
Fetal and normal Adult Hb are Isoforms, distinct forms of a protein that have the same function but are found in different tissues, i.e. a2g2 vs. a2b2
Introduction to Antibodies and Antigens
Antibodies:
-circulating glycoproteins synthesized by white blood cells (lymphocytes)
-crucial part of the body's defense system or "Immune Response"
All antibodies belong to the family of proteins called immunoglobulins, named for their role in the immune response, i.e. their ability to specifically recognize antigens (foreign invaders).
Antibody Structure:
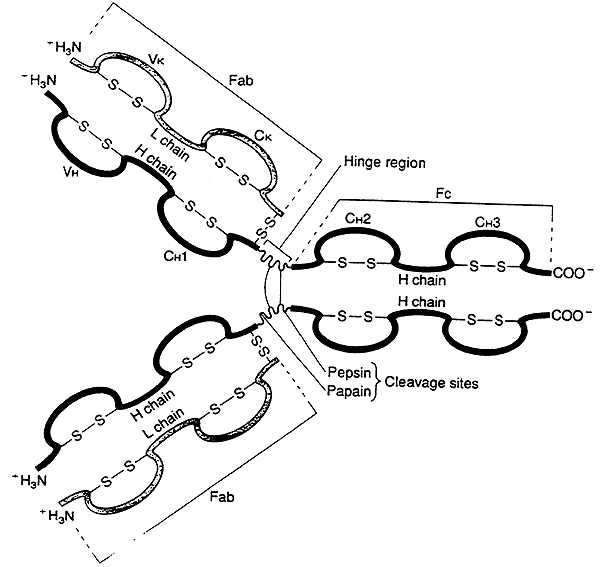
-Immunoglobulins share a common structure of three subunits arranged in a trimeric unit.
-Although, some immunoglobulins are more complex and contain multiples of this unit.
Features of the Antibody-
1) Two identical heavy chains (H).
2) Two identical light chains (L).
3) The chains are joined by interchain disulfide bonds.
4) The subunit is often depicted as a "Y".
IgG Molecule (an illustration of the basic immunoglobulin unit):
(a) several globular domains, intersecting at a central "hinge" region
(b) each globular domain is stabilized by intrachain disulfide bonds
(c) the variable (V) regions for both the heavy (H) and light (L) chains always occur together in a globular domain at the upper tips of the "Y"
(d) the two variable regions on each side form the binding site for an antigen, the immunoglobulin molecule has two identical binding sites
(e) "the two binding sites are identical within a single Ab molecule, no two Ab's are alike"
(f) the body is capable of producing millions of different variable regions
(g) the constant (C) regions within a given class of immunoglobulins remains relatively constant
"Hinge" region:
-susceptible to digestion by proteases, i.e. pepsin or papain
-enzymes cleave the immunoglobulin into two fragments:
Fab ("antigen binding") fragment contains the variable regions -----> binding sites
Fc ("crystallizable") fragment mediates many of the functions common to an immunoglobulin class.
(Fc portion is derived solely from the heavy chains, differences in the heavy chain constant regions are what distinguish one class of immunoglobulin from another.)
Antigen Recognition:
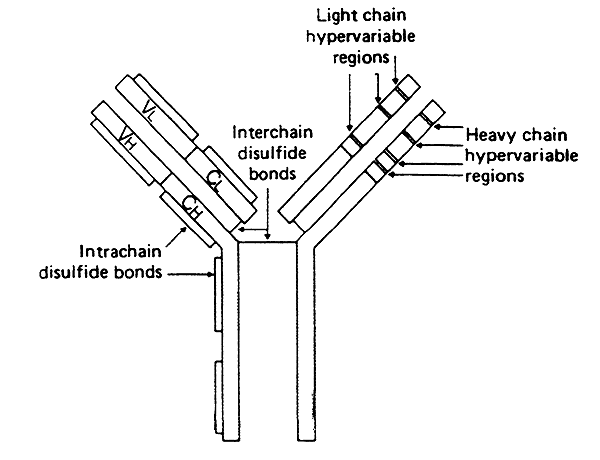
-a single antigen binding site is formed by the convergence of the variable regions from one heavy chain and one light chain (two binding sites per subunit).
-not all residues w/in the variable region change from one immunoglobulin to the next
-some do not vary much, forming the three-dimensional framework of the globular domain
-w/in each light-chain variable region are three hypervariable regions
-w/in each heavy-chain variable region are four hypervariable regions
Hypervariable Regions:
>vary tremendously forming the complementarity-determining regions (CDR's)
>in the heavy-chain variable regions there are four CDR's
>in the light-chain variable regions there are three CDR's
>the combination of seven CDR's in one binding pocket help create the vast repertoire of binding sites that form an important part of the immune system
Hapten:
>each CDR faces towards the antigen binding pocket and recognizes, but does not elicit antibody response, a small set of features on the antigen surface
>set of features is the hapten and may represent a specific chemical grouping
Types of Interactions Important in Antigen Binding:
Ionic Bonds
Salt Bridges
Hydrophobic Interactions
Hydrogen Bonding
Clinical Correlate:
Vaccines
Inject the surface component of a "dead" virus or bacteria (one that has been modified so it is non- virulent).
Ab's will be produced for this surface component.
If the vaccinated individual is later exposed to a virulent form of the virus his/her immune system will be prepared -------> making short work of the virus.
Success: chicken pox
More Difficult: HIV, has the ability to alter it's cell surface proteins in endless permutations
Clinical Correlate: Autoimmune Diseases
Body no longer recognizes "self", develops Ab's toward it's own cells.
© Dr. Noel Sturm 2014