Amino Acids/Proteins
Amino Acids:
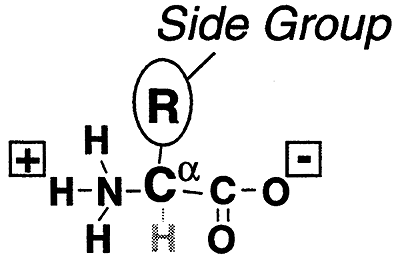
The building blocks of proteins. A protein is a polymer of amino acids.
Generic Amino Acids- (Stereochemistry...)

They can be classified by the nature of their side chain groups (R) into:
| Category | Amino Acids |
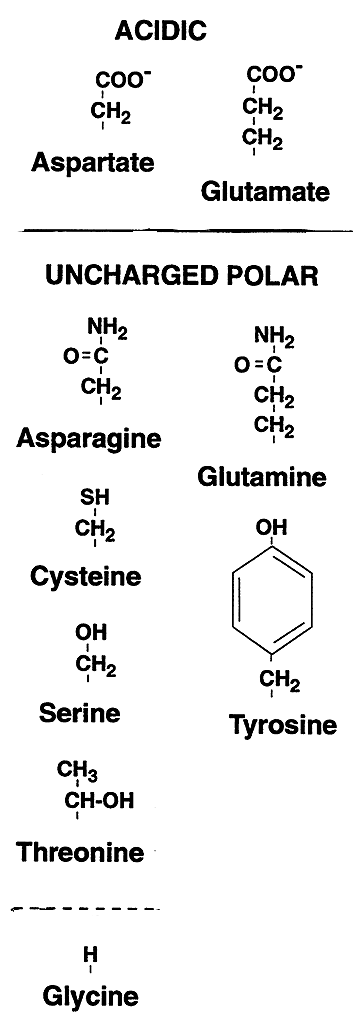
| Acidic | Aspartate (Aspartic Acid, Asp, D); Glutamate (Glutamic Acid, Glu, E) |
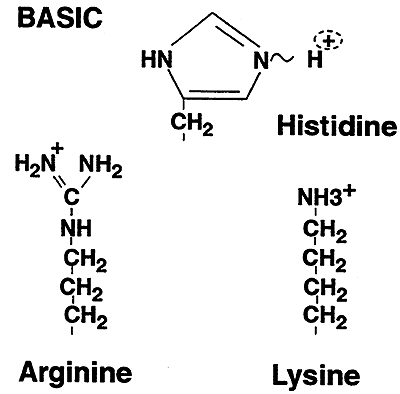
| Basic | Arginine (Arg, R); Histidine (His, H); Lysine (Lys, K) |
| Uncharged Polar (Hydrophilic) |
Asparagine (Asn, N); Glutamine (Gln, Q); Cysteine (Cys, C); Serine(Ser, S); Threonine (Thr, T); Tyrosine (Tyr, Y) |
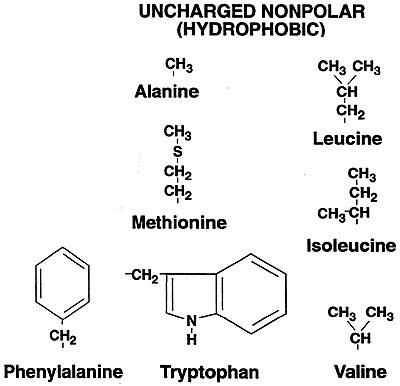
| Uncharged Nonpolar (Hydrophobic) |
Alanine (Ala, A); Isoleucine (Ile, I); Leucine (Leu, L); Phenylalanine (Phe, F); Proline (Pro, P); Tryptophan (Trp, W); Valine (Val, V); Methionine (Met, M); Glycine (Gly, G) |
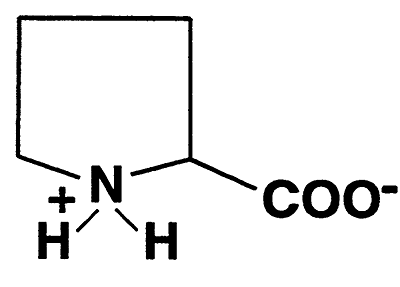
Proline
Ionization Curves:
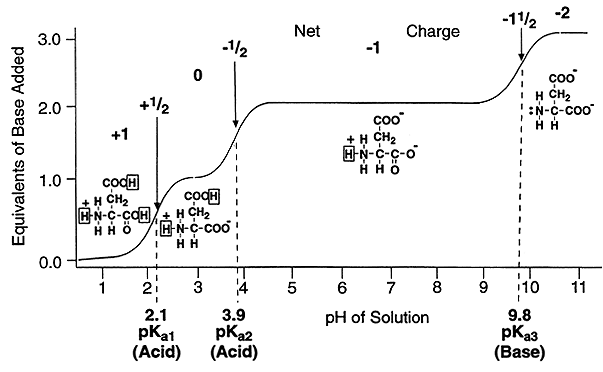
As we discussed earlier ionization curves are actually plots of the Henderson-Hasselbach equation, and are used to visualize the ionization changes that occur over a range of pH values.
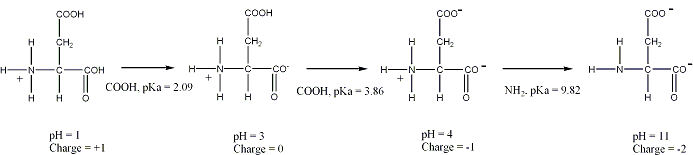
Such a plot can be used to determine the net charge on an amino acid, such as aspartic acid, at any pH value.
The Peptide Bond:
Attaches amino acids together to form a peptide. The peptide bond is repeated many times to create polypeptide chains which comprise the basic structure of all proteins.
Formation of a Peptide Bond:
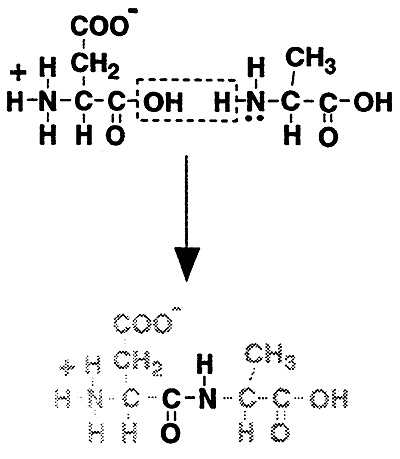
i.e. Representative Peptide:
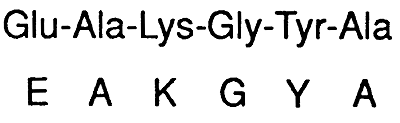
Primary Structure (10):
A particular linear sequence of amino acids unique to each protein. All share the following characteristics:
1) Amino acids in the interior of the polypeptide chain no longer posses ionizable amino or carboxyl groups. These amino acids are referred to as residues. Therefore, for a large protein, charge and polarity are determined by the side chains on the residues.
2) The end of the peptide with the free amino group, the amino terminus or N-terminus, is the beginning of the chain and is depicted on the left.
3) The end with the free carboxyl group, the carboxy terminus or C-terminus, is drawn on the right.
Secondary Structure (20):
Rotation about the two bonds attached to the a carbon allow the peptide to fold into certain three-dimensional arrangements. The two most common:
a-Helix:
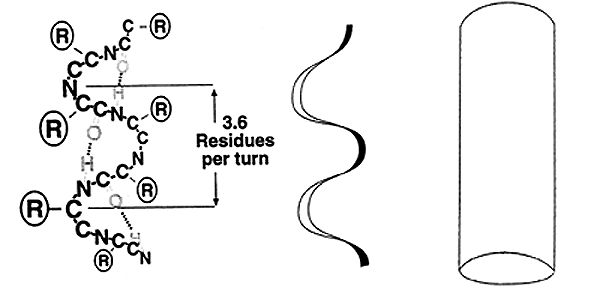
-A spiral arrangement (R groups extending outward) with ~3.6 amino acid residues per turn.
-Stabilized by intrachain H-bonds.
-Most amino acids fit well into the a-helix, with one exception Proline, known as a "helix breaker".
-Right-handed or Left-handed helix'
b-Sheet:
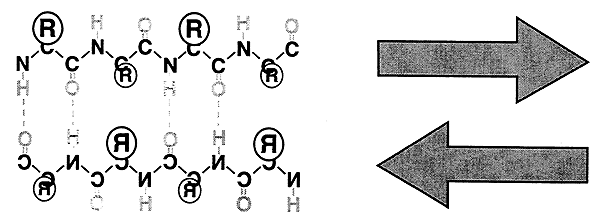
-Flat, extended arrangement.
-Interchain H-bonding.
-Parallel (same direction) or Antiparallel (opposite direction).
Tertiary Structure (30):
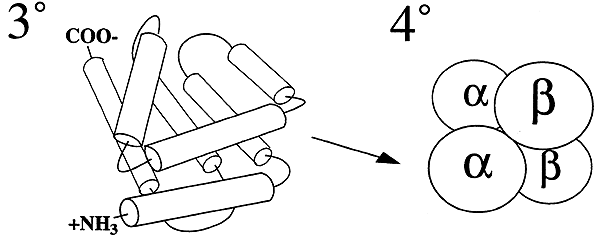
- The entire conformation of a complete polypeptide chain.
-Hydrophobic effect.
-Like Attracts Like...
Quaternary Structure (40):
-Assembly of two or more polypeptides, each having its own 20 and 30 structure, into a complete, functional protein.
Clinical Correlate:
Alpha-synuclein is a protein that is abundant in the human brain. Smaller amounts are found in the heart, muscles, and other tissues. In the brain, alpha-synuclein is found mainly at the tips of nerve cells (neurons) in specialized structures called presynaptic terminals. Within these structures, alpha-synuclein interacts with phospholipids and proteins. Presynaptic terminals release chemical messengers, called neurotransmitters, from compartments known as synaptic vesicles. The release of neurotransmitters relays signals between neurons and is critical for normal brain function.
Alpha-synuclein primary structure is usually divided in three distinct domains:
In rare cases of familial forms of Parkinson's disease, there is a mutation in the gene coding for alpha-synuclein. Three point mutations have been identified thus far: A53T, A30P and E46K. Genomic duplication and triplication of the gene appear to be a rare cause of Parkinson's disease in other lineages, although more common than point mutations. Hence certain mutations of alpha-synuclein may cause it to form amyloid-like fibrils that contribute to Parkinson's disease.
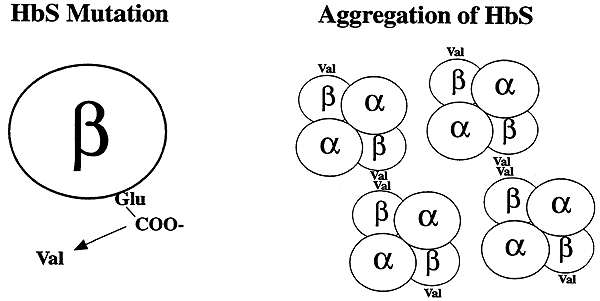
-One of the polypeptide chains making up the 40 structure of hemoglobin S undergoes a mutation.
-Glutamic Acid (Polar) to Valine (Nonpolar, Hydrophobic)
-Hydrophobic Effect.....
-Abnormal hydrophobic patch on the surface of the polypeptide. This patch mediates the aggregation of HbS molecules into long rod-like structures ("sickle" cells)