Acidosis/Alkalosis
Bases:
Have a higher affinity for protons than water and easily acquire protons in aqueous solution.
charged (+1) when protonated (Acids uncharged)
uncharged when de-protonated (Acids -1 charge)
Most common biological weak base is the amino group, -NH2
Despite the differences between acids and bases the pKa concept can be used to quantitate the relative strength of amino groups.
Notice: pKa values for carboxylic acid are less than < 7, pka values for amino groups are >7 (usually 9-11)
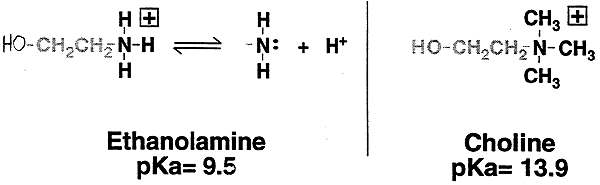
i.e. a simple biologically important 10 amine, ethanolamine, pKa = 9.5

or choline, a quaternary (40) amine, pKa = 13.9
Choline is a good compound for systems in which a permanent positive charge is desirable, i.e. membranes (hydrophilic head groups)
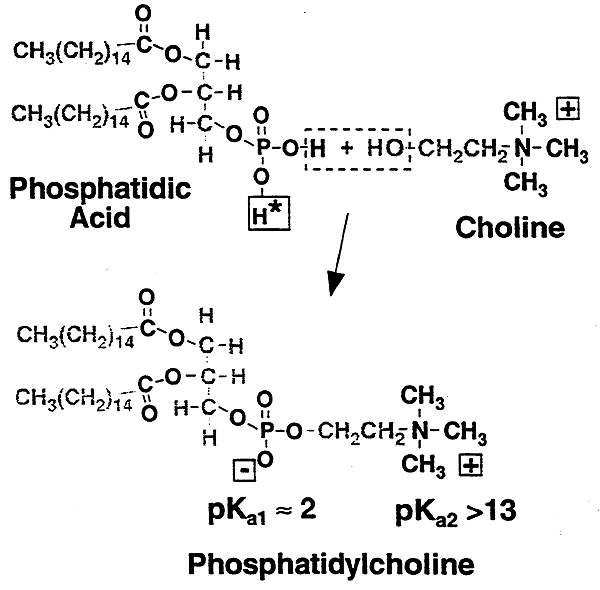
Phosphatidylcholine (lecithin)
a key amphiphilic compound in biological membranes
Buffering:
At or near their pKa both weak acids and weak bases will resist changes in pH, thus acting as buffers
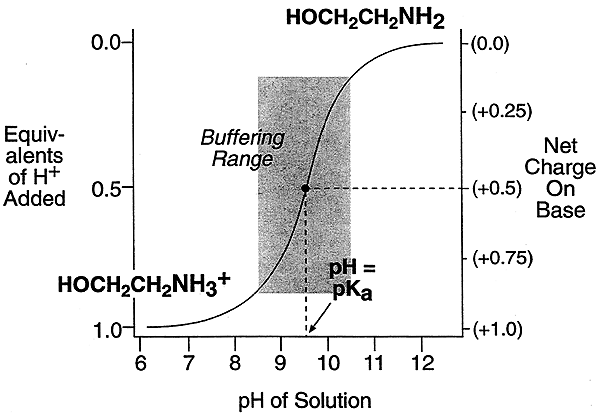
Buffering is very important in biological systems, for rapid pH changes have disastrous consequences.
The buffering capacity of ethanolamine and acetic acid occur well outside of the pH range normally seen in human blood (pH 7.35-7.45).
Thus, other ionizable compounds must serve this function in biological fluids.
The most important single buffer in human is the bicarbonate ion
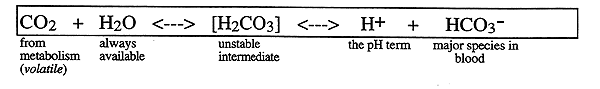
-CO2 is added to the system at varying rates by metabolic processes
-rate of formation of H2CO3 from CO2 and H2O is slow, so is enhanced by the enzyme, carbonic anhydrase, found in red blood cells (RBC)
-CO2 is expired by the lungs at varying rates (respiration)
-levels of HCO3- can be adjusted by the kidney via excretion
Acidosis/Alkalosis:
CO2Production:
-normally balanced by CO2 expired from the lungs
However, certain medical conditions can throw the equation out of balance...
Respiratory Acidosis: impaired lung capacity, with a build-up of CO2 in the lungs.
Respiratory Alkalosis: hyperventilation, net loss of CO2 from the blood.
Metabolic Acidosis: presence of excess acid in the blood (build-up of H+ from sources other than CO2), causes include ingestion of acid, production of ketoacids in uncontrolled diabetes and kidney failure.
Metabolic Alkalosis: presence of alkali in the blood (loss of H+ for reasons other than depletion of CO2), causes include ingestion of alkali, prolonged vomiting, or extreme dehydration (kidney retention of bicarbonate).
Normal (nl) bicarbonate values for blood:
pH 7.35-7.45
pCO2 35-40 mmHg
[HCO3-] 18-25 mEq/L
Compensation:
Patients are encountered who significantly deviate from normal values , yet maintain blood pH within a range compatible with life. Such patients have compensated for circumstances that would normally produce acidosis or alkalosis.
Helpful hints for recognizing acidosis/alkalosis:
1) Look at pH for first indication of either acidosis (<7.35) or alkalosis (>7.45).
2) If pCO2 varies significantly form normal in a direction opposite to pH change, then there is a respiratory defect.
3) If pCO2 varies significantly form normal in the same direction as pH, then there is a metabolic defect.
4) Compensation involves pCO2 and HCO3- varying in the same direction.
Respiratory Defects:
Respiratory Acidosis- retain HCO3-, secrete H+
Respiratory Alkalosis- excrete HCO3-, retain H+
Metabolic Defects:
Metabolic Acidosis- excrete CO2
Metabolic Alkalosis- retain CO2
© Dr. Noel Sturm 2014