OXIDATION OF CYCLOHEXANOL TO CYCLOHEXANONE
Equation:
NaOCl + CH3CO2H + C6H11OH --> C6H10O + HCl + CH3CO2-Na+ + H2O
Tabled Amounts of Reactants Used and Amounts of Products Theoretically Possible
|
Compound |
C6H11OH |
NaOCl |
CH3COOH |
C6H10O |
|
MW |
100 |
74.5 |
60.0 |
98.0 |
|
Moles |
|
|
|
|
|
Grams |
|
|
|
|
|
density |
0.962 |
- |
1.049 |
0.998 |
|
ml |
10.4 |
90.0 |
25.0 |
|
|
Concentration |
- |
10g/dl |
17.5M |
- |
1. Place a 250 mL Erlenmeyer flask atop your hotplate stirrer. Add a magnetic stirring bar and your glass thermometer to the flask. Put your small support ring over the flask. Use the ring arm to support your thermometer.
2. Add 10.4 mL cyclohexanol and 25 mL glacial acetic acid into the flask.
3. Add 90-mL NaOCl (10% w/v) to uour 250 mL separatory funnel.
4. Prepare an ice-water bath and place it next to your hotplate stirrer.
5. Begin stirring the contents of the flask and start adding the NaOCl from your dropping funnel dropwise to the reaction flask. This addition will take 25-45 mins.
6. Maintain the temperature of the reaction mixture at 30 - 35 oC by controlling the rate of addition of NaOCl and the use of your ice - water bath, whenever necessary. Do not over-cool your reaction mixture.
7. After the completion of the NaOCl addition, the reaction mixture should be a greenish-yellow and should give a positive test with KI-starch paper (i.e. placing a drop of your reaction mixture onto the test strip should turn the paper blue or black ).
8. If the mixture is not greenish-yellow or does not maintain a greenish-yellow color during subsequent stirring, add sufficient NaOCl (1 - 3-mL) to impart a greenish-yellow color and yield a positive KI-starch test.
9. Remove the cooling bath and stir for fifteen minutes at room temperature. Add solid NaHSO3 in small portions, until the reaction mixture turns colorless and gives a negative KI-starch test.
10. Pour the contents of your reaction flask into a separatory funnel, add 5 g of solid NaCl, swirl, allow layers to separate, and drain off the lower aqueous layer.
11. Wash the organic layer with two - 10 mL aliquots of saturated NaHCO3.
12. Drain off the lower aqueous layer and transfer the ketone to a 50 mL Erlenmeyer flask and dry the
product over anhydrous MgSO4.
13. Decant the liquid sitting on top of the MgSO4 into another tared 50 mL Erlenmeyer flask. Weigh the
Erlenmeyer flask, containing the solution, with a stopper in it. Record the weight in your notebook and
make sure it is recorded in the lab report. Save your product for the Carbonyl tests.
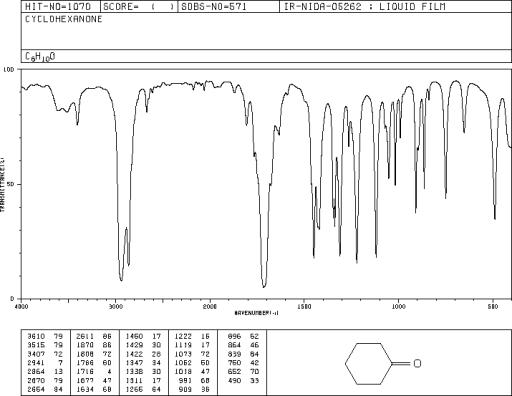
Laboratory Report. Supply the usual, minus the boiling range and B.P., and determine the OH and C=O peaks by looking at the IR spectra on the following pages.
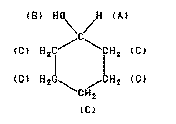
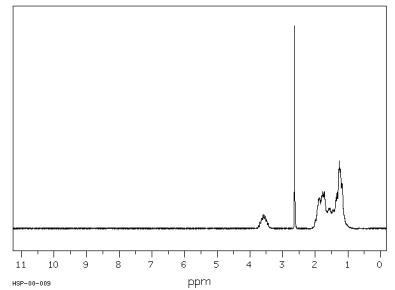
Cyclohexanol Spectra
Assign. Shift(ppm)
A 3.58
B 2.63
C 1.04 to 2.04
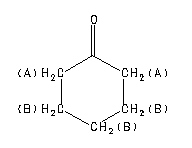
Cyclohexanone Spectra
Assign. Shift(ppm)
A 2.35
B 2.07 to 1.55
Explanation of Procedure
Steps 5-6 HOCl is being generated and is immediately used to oxidize cyclohexanol to cyclohexanone.
Step 6 Above 35oC, "-halogenation of a ketone can occur.
Remember HCl+ + HOCl W Cl2 + H2O
Step 8 If Cl2 is present with HOCl, it is a sign that the alcohol was the limiting reactant. If NO excess of Cl2 W HOCl present, you don't know if the alcohol was the limiting reactant or not.
The reaction is
Cl2 + 2KI ----> 2KCl + I2.
I2 + starch ----> starch-I2 (blue black).
Step 9 Cl2 must be removed now, otherwise it will come out into the room during distillation. Adding too much NaHSO3 will cause the excess to ----> SO2 & enter the room, making you & your colleagues leave.
Step 10 To "salt-out" the product. (Che 301)
Step 11 CH3COOH + NaHCO3 ---> CH3COO-Na+ + [H2CO3]
[H2CO3] ---> CO2 + H2O
Step 14 MgSO4 + nH2O ---> MgSO4 . nH2O (s)
HOMEWORK
1. Why are NaOCl and Glacial Acetic Acid used in the preparation of Cyclohexanone?
2. Write the chemical equation for the oxidation of cyclohexanol.
3. Why was NaHSO3 used?