Steam
Distillation
The
purpose of this experiment is to demonstrate the ability to distill an organic
liquid, limonene, at a temperature considerably below that of the boiling
point of the pure liquid.
Procedure
Construct a steam distillation apparatus as below.

Add your pulp and water to the distillation pot (250 mL rb flask) UNTIL HALF FULL.
Add
your magnetic stirring bar and start the mixture stirring.
Heat the mixture to a boil.
Note: Attempt to control the rate of heating so that excessive foaming does not occur.
At
the first drop of distillate, record
temperature and volume of distillate collected.
Repeat this every four mL, until 50 mL of distillate has been collected.
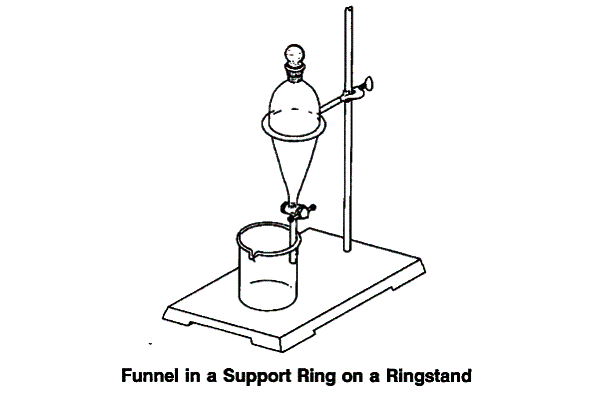
Stop the distillation. The distillate should have two layers. The top layer should be oil (limonene) and the bottom one should be aqueous. Transfer the distillate to a separatory funnel and allow the mixture to stand for 5 - 10 minutes to allow complete separation. (See Figure above).
Drain
off the aqueous layer.
Draw
a Temperature vs Volume graph of your lab data in your lab report.
Test
for unsaturation (multiple bonds between two carbon atoms):
a) Place one drop of cyclohexane in a depression of a white spot plate and add 1 drop of KMnO4.
A purple color should be observed. There is no reaction. Stir the solution with a stirring rod and
spot the solution onto a piece of filter paper. A purple spot should be visible on the filter paper.
b) Place one drop of cyclohexene in a depression of a white spot plate and add 1 drop of KMnO4.
A red-brown color should form. Stir the solution with a stirring rod and spot the solution onto a
piece of filter paper. A brown spot should be visible on the filter paper.
c) Place one drop of your distilled oil in another depression of a white spot plate, and add 1 drop
KMnO4. red-brown color should form. Stir the solution with a stirring rod and spot the solution
onto a piece of filter paper. A brown spot should be visible on the filter paper.
Turn
in your product in an appropriately labeled narrow mouth bottle.
==============================================
Disposal
of Spent Citrus Peel
==============================================