Preparation of 2-chloro-2-methylbutane
The reaction is SN1. The overall reaction is:
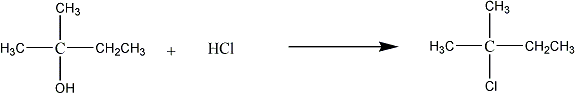
CAUTION:
Wear gloves during the entire experiment
Procedure
Take your 125 mL separatory funnel and stopper to hood #6 ( Workstations 12 & 13 ).
Add 10mL. of 2-methyl-2-butanol and 25mL of concentrated (12M) HCl to the sep. funnel,
Stopper, and take the funnel to your hood workstation..
Remove
the stopper from the funnel and swirl the contents of the separatory funnel
gently,.
After swirling for about 1 minute, stopper and invert the funnel.
After inverting, vent to release excess pressure by carefully opening the stopcock.
Shake, and vent the funnel intermittently for about 5 minutes.
Allow
the contents of the funnel to stand until the mixture has separated into two
distinct layers. If two layers
do not separate, consult your instructor.
Separate the layers and pour the aqueous layer into a 500-mL Erlenmeyer flask.
Wash the organic layer with 10mL of water.
Allow
the contents of the funnel to stand until two distinct layers form. Again,
if two layers
Separate the layers and add the aqueous layer to your 500-mL Erlenmeyer flask.
Place the organic layer into your separatory funnel, and add to it 10mL of 5% sodium bicarbonate.
Swirl the contents of the separatory funnel gently, without the stopper in the funnel.
After swirling for about 1 minute, stopper and invert the funnel.
After inverting, vent to release excess pressure by carefully opening the stopcock.
Shake, and vent the funnel intermittently for about 5 minutes.
Allow the contents of the funnel to stand until the mixture has separated into two distinct layers. If two layers do not separate, consult your instructor.
Separate the layers and add the aqueous layer to your 500-mL Erlenmeyer flask.
Wash the organic layer with 10mL of H2O.
Allow the contents of the funnel to stand until the mixture has separated into two distinct layers. If two layers do not separate, consult your instructor.
Separate the layers and add the aqueous layer to your 500-mL Erlenmeyer flask.
Transfer the organic layer to a 50-mL Erlenmeyer flask and add anhydrous CaCl2 .
Swirl the flask, intermittently for 15 minutes.
Transfer your product to a tared sample bottle, appropriately labeled.
Turn in your 2-chloro-2-methylbutane and 1-bromobutane products.
Notes
The reaction, including mechanism is:
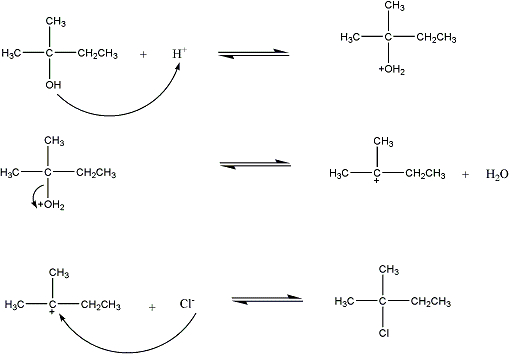
Venting is necessary because HCl has a high vapor pressure.
The washing with water is to remove any excess HCl.
The washing with NaHCO3 is to neutralize the acid that is still present, ( i.e. HCl + NaHCO3 6 NaCl + H2CO3, which immediately disintegrates to CO2 and water. Because CO28 gas is produced, venting is necessary.