Preparation
of 1-Bromobutane
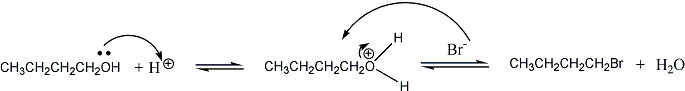
The mechanism is SN2. The overall reaction is:
H2SO4 + NaBr + CH3CH2CH2CH2OH -------> CH3CH2CH2CH2Br + H2O + NaHSO4
CAUTION:
Wear gloves during the entire experiment
At your hood workstation, take a 100-mL round-bottomed flask, and clamp the flask to the ringstand attached to your hotplate/stirrer..
Add 17 gm. of NaBr, 15 mL H2O, and 10 mL 1-butanol to the flask and begin stirring the solution with the magnetic stirrer.
S L O W L Y AND CAREFULLY add 15mL conc. H2SO4 to the flask.
Equip
the flask with a reflux condenser and begin
circulating water through it - water going in the bottom and out
the top of the condenser. (
see the picture on the next page )
Heat the flask gently (using the hot plate at a setting of about “4” ).
Continue gentle heating until the mixture begins to reflux.
Once
this is observed, continue "refluxing" the mixture for 60 minutes,
controlling the

Once the contents of the flask ceases to bubble, remove the reflux condenser, unclamp the flask from the ringstand and pour the contents of the flask into the Separatory funnel.
Add 700 mL of water to your 1L waste beaker.
Separate the lower aqueous layer from the organic layer and add it to the contents of your 1 L Beaker, WITH STIRRING. ( Why is the aqueous layer on the bottom ? )
Extract
the organic layer with 15 mL H2O.
Separate
the UPPER aqueous layer from
the organic layer and add it to the contents
Extract the organic layer with 15mL saturated
sodium bicarbonate.
Drain
the lower ORGANIC layer into a DRY 50 mL Erlenmeyer flask, and add
CaCl2 .
Swirl the flask occasionally for a period of 5 minutes. ( Why was the organic layer now on the bottom ? )
Allow
the drying agent to settle and DECANT the liquid into a
TARED sample bottle, appropriately labeled.
Save it in your locker for the alkyl halide tests.
Notes:
The
reaction, with mechanism is:
Refluxing is a means of trapping the vapors of the reactants and cooling them enough to return to the reaction flask. There, they have another opportunity to react to form 1-bromobutane
Washing with water simply removes any unreacted H2SO4 molecules.
Washing with saturated NAHCO3 removes any remaining H2SO4.molecules.
The water is removed by CaCl2, as described
in the propanoic acid experiment.