Alkene Classification Tests
Two common types of unsaturated compounds are alkenes and alkynes, characterized by the carbon-carbon double and triple bond, respectively, as the functional group. There are no simple direct ways to prepare solid derivatives of unsaturated aliphatic compounds having no other functional groups. Two common qualitative tests for unsaturation are the reaction of the compounds with bromine in carbon tetrachloride and with potassium permanganate. In both cases a positive test is denoted by decolorization of the reagent.
A. Bromine in Carbon Tetrachloride Bromine will add to the carbon-carbon double bond of alkenes to produce dibromoalkanes and with alkynes to produce tetrabromoalkanes. When this reaction occurs, molecular bromine is consumed, and its characteristic dark red‑brown color disappears if bromine is not added in excess. The rapid disappearance of the bromine color is a positive test for unsaturation.
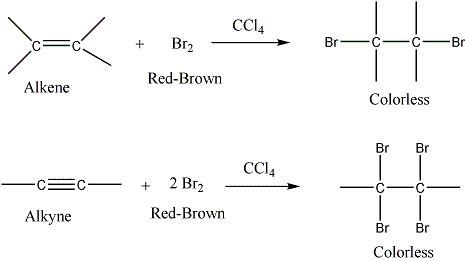
The test is not unequivocal, however, because some alkenes do not react with bromine, and some react very slowly. In the case of a negative test, therefore, the potassium permanganate test should be performed.
B. Potassium Permanganate (the Baeyer Test) A second qualitative test for unsaturation, the Baeyer test, depends on the ability of potassium permanganate to oxidize the carbon‑carbon double bond to give alkanediols or the carbon-carbon triple bond to give carboxylic acids
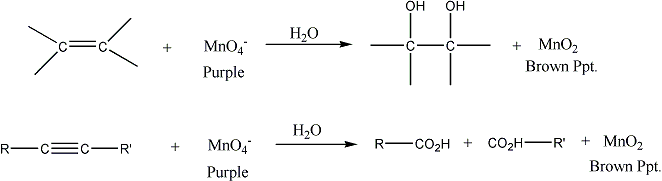
The permanganate is destroyed
in the reaction, and a brown precipitate of MnO2 is produced.
The disappearance of the characteristic color of the permanganate ion
is a positive test for unsaturation. However, care must be taken, since compounds
containing certain other types of functional groups (for example, aldehydes,
containing the --CH=O group) also decolorize permanganate ion.
EXPERIMENTAL:
In a test tube, dissolve 1 or 2 drops of cyclohexane in 20 drops 95% ethanol, then add 0.1M potassium permanganate solution drop-wise, swirling the test tube from time to time, and observing the results. Count the number of drops added before the purple permanganate color persists. (To test for permanganate color; with your stirring rod, place a drop of solution from your test tube onto a piece of filter paper. The appearance of a purple ring around the outer edge of your spot indicates the presence of permanganate.) Repeat this process with cyclohexene, and your unknown. A significant difference in the number of drops used in your cyclohexane test versus the number used in your cyclohexene and unknown tests constitutes a positive test for un-saturation. You do not have to reach the permanganate color end point with cyclohexene or your product, just be sure that ten (10) more drops were added to these tubes than was added to the cyclohexane tube.
Dispose of the contents
of the test tubes into the “Recovered Organic Solvents” bottle.
Failure to do so will result in a 2 point deduction from your score